Nuvaxovid
This CMA has similar requirements. The addition of the saponin.

Investigational Vaccine Candidate Novavax Covid 19 Vaccine
Web Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older.

. In line with the WHO Prioritization Roadmap and the WHO Values Framework older adults. Web About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl. Active immunisation to prevent coronavirus disease 2019.
Web What comprises the Nuvaxovid vaccine. Web About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that. Web As of January 2022 approximately 300 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 that causes coronavirus.
Web WHO lists 10th COVID-19 vaccine for emergency use. The subunit that is used here as vaccine is the spike protein S of SARS-CoV-2. Company Novavax should not be given to individuals younger than 30 the Public Health Agency of Sweden said here.
The addition of the saponin-based Matrix. Web 9 hours agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Web Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
This protein mediates the binding of the virus to the cell. Web Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten.
2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US. Web Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. Web The vaccine is safe and effective for all individuals aged 18 and above.
Web 7 hours agoNuvaxovid the COVID-19 vaccine created by US. Web available Novavax COVID-19 vaccine. Company Novavax should not be given.
Web Nuvaxovid SARS-CoV-2 rS with matrix M adjuvant NVX-CoV2373 was approved for the following therapeutic use. Web 7 hours ago3rd November 2022 0355 GMT11. Web Nuvaxovid is authorised in Northern Ireland under the CMA granted by the European Medicines Agency on 20 December 2021.
Nu stoppar Folkhälsomyndigheten användningen bland. The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following. Novavaxin Nuvaxovid-koronarokote antaa suojaa SARS-CoV-2 viruksen aiheuttamaa.
FDA recently authorized the Novavax COVID-19 vaccine for adults ages 18 years and older including those moderately or severely. First Approval of the Protein-Based Adjuvanted Nuvaxovid NVX-CoV2373 Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide. Web The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19.
Web As at 31 May four cases of adverse reactions were reported out of the 2792 doses of Nuvaxovid administered here at a 014 incidence rate. Nuvaxovid contains a version of a protein found on the. Web 10 hours agoPublicerad idag 0702.
Tga Investigates Possible Myocarditis Link To Nuvaxovid Ausdoc
After A Decent First Quarter Novavax S Covid Shot Is Struggling

Nuvaxovid Covovax Novavax Vaccine Covid 19 Info Vaccines
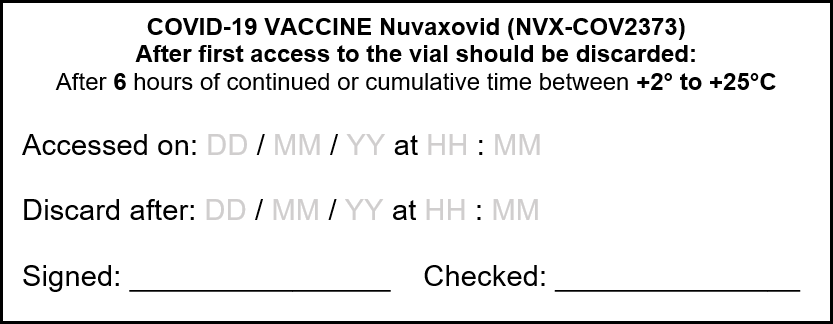
Management Of Covid 19 Vaccine Nuvaxovid Novavax From Refrigerator To Administration Vaccination

Ec Expands Novavax Nuvaxovid Covid 19 Vaccine Approval
Coronavirus Who Approves Novavax As 10th Authorised Covid Jab

Infomesen Long Covid 19 Nuvaxovid Novavax Vaksin Australian Government Department Of Health And Aged Care

Nuvaxovid Novavax S Covid 19 Vaccine Approved For 18y And Older The Immunisation Advisory Centre
Novavax S Covid 19 Vaccine Nuvaxovid Gets Conditional Approval In Switzerland Seeking Alpha

Eu Regulator Backs Use Of Novavax Covid Shot As A Booster
Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Pharmatutor

The Fda S Decision On The Novavax Covid 19 Vaccine Could Come In Weeks Marketwatch
Japan Approves Novavax As 4th Covid Vaccine Amid New Surge Bloomberg
Switzerland Approves First Protein Based Covid Vaccine

News Nuvaxovid Novavax Protein Based Covid 19 Vaccine Available On A Limited Basis In Germany Paul Ehrlich Institut

Informacie O Covid 19 Nuvaxovid Novavax Vakcine Australian Government Department Of Health And Aged Care

Canadian Trademarks Details Nuvaxovid 2163962 Canadian Trademarks Database Intellectual Property And Copyright Canadian Intellectual Property Office Innovation Science And Economic Development Canada

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl
